Acid-alkali status is increasingly relevant as we tend more towards acid-forming diets.1 Modern western diets have a higher content of acid- forming foods as opposed to base (alkaline)-forming foods, and this results in higher acidity in the body along with the development and progression of chronic health conditions.2
In sports medicine, the process of alkalisation can increase an athlete’s capacity for high-intensity exercise.1 In clinical medicine, several renal diseases, such as kidney stones and kidney failure, require both control and manipulation of acid-base status to improve clinical outcomes.1 Cardiovascular diseases such as hypertension have also been associated with an altered acid–base balance.2 Diet is a powerful way of influencing human acid-alkali status.1
How can we determine if a certain food is alkalising or not?
The acid loads of foods or diets are determined by a well-established calculation method called the potential renal acid load (PRAL).4 PRAL is a way to evaluate a food’s acidifying or alkalising potential.4 The PRAL calculation is physiologically based and takes into account different intestinal absorption rates of individual nutrients such as sulfur-containing protein, as well as the amount of sulfate produced from metabolised proteins.4 The PRAL method was experimentally validated in healthy adults and it showed that, under controlled conditions, acid loads and renal net acid excretion (NAE) can be reliably estimated from dietary composition.4
Factors such as the decline in renal function that occurs with ageing may increase the risk of metabolic acidosis.4 The pathogenesis of metabolic acidosis involves either a net gain of acid (hydrogen ions represented by H+) or a net deficit of base (bicarbonate ions represented by HCO3–) from the extracellular fluid.6 Simply put, higher numbers of hydrogen ions than bicarbonate ions lead to metabolic acidosis.
In the medical field, metabolic acidosis can be calculated by the anion gap.9 The anion gap is the difference between the unmeasured anions (negatively charged ions) and the unmeasured cations (positively charged ions).9 A high anion gap value means that your blood is more acidic than normal.
A diet that contains alkaline forming foods is highly important in maintaining acid-alkai balance.
It’s all about the Chemistry
Different forms of minerals effect different chemical reactions in the body.
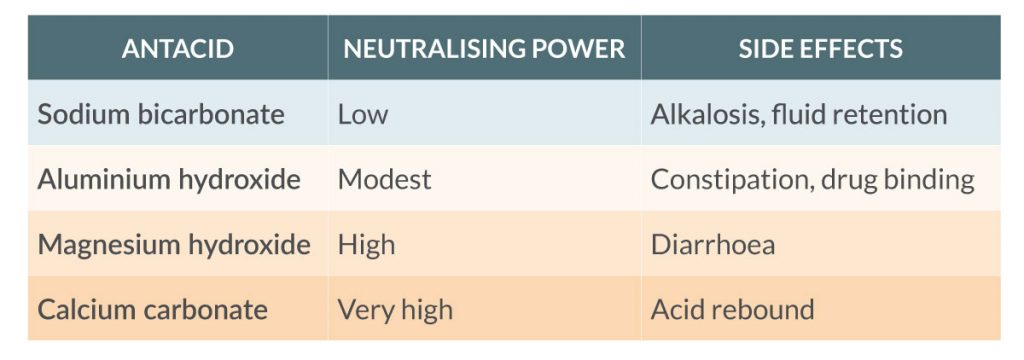
For example: Calcium carbonate (chalk) is a common, medically prescribed and very powerful antacid with strong potential to cause acid rebound effects.9 The reason is purely chemical. We call upon our endogenous buffering systems to balance the acidity created by hydrogen ions. However, in the stomach where we want a more acidic environment, carbonate ions from calcium carbonate supplementation can accept two hydrogen ions which increases gastric pH more dramatically than sodium bicarbonate which only accepts one hydrogen ion with much less impact on gastric pH.
Fewer citrate forms = less alkalising potential
Magnesium carbonate readily dissociates in the acidic environment of the stomach into magnesium oxide and carbon dioxide. The carbonates are swiftly eliminated, which means they do not make it into the systemic circulation to take part in the body’s buffering systems. We are left with just the mineral itself for alkalising effect in a less desirable, less bioavailable form.10
Citrates for the win
Potassium, magnesium and calcium are known as alkaline earth minerals simply due to their position on the periodic table. When combined with citrate molecules in supplemental form the chemistry shows that they offer the strongest alkalising effect. Citrate ions, in an acidic environment (the stomach), can accept three hydrogen ions to form citric acid and hydroxyl ions which has an overall alkalising effect on the blood.
The form of alkalising mineral you take is very important for long term outcomes. At True Medicine we assess your individual needs and only use quality and well researched supplements.
References
- Remer, T., Dimitriou, T. & Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am. J. Clin. Nutr.77, 1255–1260 (2003).
- Frassetto, L., Banerjee, T., Powe, N. & Sebastian, A. Acid balance, dietary acid load, and bone effects-a controversial subject. Nutrients10, 1–9 (2018).
- Hasan, A. Handbook of blood gas/acid-base interpretation: Second Edition. Handbook of Blood Gas/Acid-Base Interpretation: Second Edition (2013). doi:10.1007/978-1-4471-4315-4.
- Fakhri, Y. et al. Association between sodium bicarbonate consumption and human health: A systematic review. Int. J. Med. Res. Heal. Sci. 5, 22–29 (2016).
- Verove, C., Maisonneuve, N., El Azouzi, A., Boldron, A. & Azar, R. Effect of the correction of metabolic acidosis on nutritional status in elderly patients with chronic renal failure. J. Ren. Nutr. 12, 224–228 (2002).
- Adeva-Andany, M. M., Fernández-Fernández, C., Mouriño-Bayolo, D., Castro-Quintela, E. & Domínguez-Montero, A. Sodium bicarbonate therapy in patients with metabolic acidosis. Sci. World J. 2014, (2014).
- Gregory, N. S. et al. Potassium citrate decreases bone resorption in postmenopausal women with osteopenia: A randomized, double-blind clinical trial. Endocr. Pract. 21, 1380–1386 (2015).
- Peart, D. J., Siegler, J. C. & Vince, R. V. Practical recommendations for coaches and athletes: Ameta-analysis of sodium bicarbonate use for athletic performance. J. Strength Cond. Res. 26, 1975–1983 (2012).
- Maton, P. N. & Burton, M. E. Antacids Revisited. A Review of Their Clinical Pharmacology and Recommended Therapeutic Use. Drugs 57, 855–870 (1999).
- Lindberg JS, Zobitz MM, Poindexter JR, P. C. Magnesium bioavailability from magnesium citrate and magnesium oxide. J Amer Coll Nutr 9, 48–55 (1990).